Filter
1098
Text search:
specimen
collection
Featured
167
353
Language
Document type
658
221
96
51
34
15
10
9
2
1
1
Countries / Regions
94
41
39
38
38
35
28
26
25
23
23
23
19
18
18
15
14
13
13
13
12
11
10
9
9
7
7
6
6
6
5
5
5
5
5
4
4
4
4
4
4
4
4
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
Publication Years
Category
499
134
56
43
18
15
12
Toolboxes
128
124
104
82
57
57
36
16
15
15
13
11
10
8
7
4
4
4
3
3
1
This document provides a systematic approach in developing a coordinated, standardized, reliable, efficient, cost-effective, and sustainable specimen transport and referral system to support IVHD and VL testing networks. This document provides techn
...
This guide includes information relevant for tuberculosis (TB) program and laboratory managers, as well as Ministry of Health officials across disease programs interested in establishing integrated solutions for specimen referral. Though TB-focused
...
The main objectives of these guidelines are to:
1. contribute to the quality assurance of medicinal plant materials used as the source for herbal medicines to improve the quality, safety and efficacy of finished herbal products; 2. guide the formulation of national and/or regional GACP guideli ...
1. contribute to the quality assurance of medicinal plant materials used as the source for herbal medicines to improve the quality, safety and efficacy of finished herbal products; 2. guide the formulation of national and/or regional GACP guideli ...
Improve identification, verification, communication and coordination.
Antimicrobial Resistance Surveillance and Research Network | This manual describes well accepted methods to carry out drug susceptibility testing on important gram positive and gram negative clinically relevant bacteria. Methods of specimen
...
HIV-1 drug resistance (HIVDR) genotyping is an essential component of the WHO global HIVDR surveillance strategy. Plasma “gold standard” specimen type for HIVDR genotyping, but its use may not be feasible in rural, remote areas in low- and middl
...
UNAIDS/WHO Working group
HIV/AIDS and STI surveillance 2015 / Reference
SOP number: ICMR-NIV/2019-nCoV/Specimens_01
Prepared by: Dr. Y.K. Gurav Reviewed by: Dr. V. Potdar Approved by: Dr. P. Abraham
Date: 19/01/2020 Date: 20/01/2020 Date: 20/01/2020
Prepared as an outcome of ICMR Expert Group on Immunophenotyping of Hematolymphoid Neoplasms | Coordinated by Division of Non Communicable Diseases | This document addresses on various issue related to good quality practices in laboratory work up of flow cytometric immunophenotyping and will be of u
...
Available in Arabic, Chinese, English, French, Portuguese and Spanish
https://apps.who.int/iris/handle/10665/334254
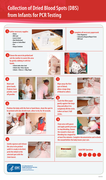
This clinical job aid provides health care workers with information on how to collect specimens for early infant diagnosis on dried blood spots, as well as drying and packaging for transport.
For SARS-CoV-2 testing only
The Mapping Antimicrobial Resistance and Antimicrobial Use Partnership (MAAP) project has conducted a multi-year, multi-country study that provides stark insights on the under-reported depth of the antimicrobial resistance (AMR) crisis across Africa and lays out urgent policy recommendations to addr
...
The National Guidelines for HIV-1 Viral Load Laboratory Testing support plans to scale up viral load (VL) testing to reach the 90-90-90 targets in India. This phased scale-up includes the setup of 70 additional VL testing laboratories nationally. These guidelines include laboratory design considerat
...