Filter
21
Text search:
nirmatrelvir
Featured
11
Language
Document type
11
7
2
1
Countries / Regions
3
1
Authors & Publishers
Publication Years
Category
3
1
1
Toolboxes
20
WHO has updated its guidelines for COVID-19 therapeutics, with revised recommendations for patients with non-severe COVID-19. This is the 13th update to these guidelines.
Updated risk rates for hospital admission in patients with non-severe COVID-19
The guidance includes updated risk rates for
...
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir
...
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir
...
Nirmatrelvir-ritonavir for COVID-19
recommended
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir
...
This twelfth version of the WHO living guideline now contains 19 recommendations. This latest update provides updated recommendations for remdesivir, addresses the use of combination therapy with corticosteroids, interleukin-6 (IL-6) receptor blockers and Janus kinase (JAK) inhibitors in patients wi
...
This report’s central premise is that diagnostics and therapeutics, and associated test to treat strategies, are fundamental components of the pandemic response, both for COVID-19 and for future health threats. Two years into the COVID-19 pandemic, this report reflects on the main challenges and k
...
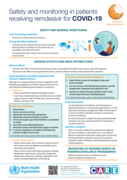
Administration of Remdesivir for COVID-19
recommended
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir
...
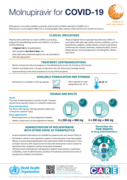
Molnupiravir for COVID-19
recommended
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir
...
Administration of Molnupiravir for COVID-19
recommended
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir
...
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir
...
Remdesivir for COVID-19
recommended
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir
...
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir
...
Ongoing Living Update of COVID-19 Therapeutic Options: Summary of Evidence, Rapid Review 22 February 2022
recommended
This document includes the results of a rapid systematic review of current available literature. The information included in this review reflects the evidence as of the date posted in the document. In recognition of the fact that there are numerous ongoing clinical studies, PAHO will periodically up
...
26 de abril del 2022. La Organización Panamericana de la Salud presenta estas consideraciones con el fin de apoyar la toma de decisiones relativa al manejo de pacientes con COVID-19 en la Región de las Américas. Las recomendaciones tienen en cuenta la evidencia más reciente, el estado de vacunac
...
Globally each year, millions of people suffer illness or lose their lives because the vaccines, medicines and diagnostic tests that they need are either unavailable or unaffordable – and this lack of access to medicine is acute in low- and middle-in-
come countries (LMICs). While the COVID-19 pan
...
May 9, 2022.Since the onset of the COVID-19 pandemic, a large number of clinical trials have been planned and developed to assess the effectiveness and safety of various interventions that could prevent hospitalizations and progression to severe disease in people infected with SARS-CoV-2. Currently,
...
21 January 2022
The overall threat posed by Omicron largely depends on four key questions: (i) how transmissible the variant is; (ii) how well vaccines and prior infection protect against infection, transmission, clinical disease and death; (iii) how virulent the variant is compared to other varian
...
The World Health Organization (WHO) recognizes the challenges countries face for maintaining their COVID-19 response while addressing competing public health challenges, conflicts, climate change and economic crises. WHO continues to support countries in adjusting COVID-19 strategies to reflect succ
...
The objective of Health in the Americas: Overview of the Region of the Americas in the Context of the COVID-19 Pandemic is to respond to the need to address important public health issues in an increasingly timely manner, while serving as a platform with a close focus on specific issues of regional
...
Les notes d’orientation décrivent les mesures essentielles que les décideurs nationaux et infranationaux peuvent mettre en place concernant les aspects suivants de la lutte contre la COVID-19 : les tests de diagnostic, la prise en charge clinique, la réalisation des cibles en matière de vaccin
...