Filter
186
Featured
20
82
Language
Document type
140
18
16
4
3
2
1
1
1
Countries
20
10
9
8
8
7
7
6
5
5
5
3
3
3
3
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
Publication Years
Category
112
11
8
8
3
2
1
Toolboxes
38
4
3
3
3
3
3
3
2
2
1
1
1
HIV-1 drug resistance (HIVDR) genotyping is an essential component of the WHO global HIVDR surveillance strategy. Plasma “gold standard” specimen type for HIVDR genotyping, but its use may not be feasible in rural, remote areas in low- and middle-income countries, since preparing and storing it
...
Toolkit
HIV Treatment and Care
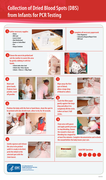
This clinical job aid provides health care workers with information on how to collect specimens for early infant diagnosis on dried blood spots, as
...
This job aid provides information for laboratorians about how to receive, process, and store dried blood spot specimens collected for early infant diagnosis, viral load, or drug resistance testing.
The updated List of Essential Diagnostics contains 46 general tests that can be used for routine patient care as well as for the detection and diagnosis of a wide array of disease conditions, and 69 tests intended for the detection, diagnosis and monitoring of specific diseases.
The List is divid
...
This manual describes some of the strategic, managerial, financial, technical and scientific aspects to be considered in establishing a national EQA programme for clinical laboratories and other testing services at all health care levels
This document provides a systematic approach in developing a coordinated, standardized, reliable, efficient, cost-effective, and sustainable specimen transport and referral system to support IVHD and VL testing networks. This document provides technical and programmatic recommendations on the approp
...
Option B+
Journal of The Association of Physicians of India, Vol. 63 November 2015,, pp.77-96
This user guide is designed to provide national malaria control programmes with general information on glucose-6-phosphate dehydrogenase (G6PD) deficiency. Individuals with this condition may be at risk of adverse effects from medicines commonly used to cure Plasmodium vivax malaria, as well as from
...