Filter
2128
Filtered Results: 2128
Text search:
Euroepan
Union
Featured
Recommendations
141
New Publications
497
Language
Document type
No document type
1106
Studies & Reports
544
Guidelines
195
Manuals
85
Strategic & Response Plan
69
Fact sheets
49
Situation Updates
28
Training Material
27
Resource Platforms
12
Brochures
6
Online Courses
3
Infographics
2
Dashboards/Maps
1
Videos
1
Countries / Regions
Western and Central Europe
169
Global
98
Ukraine
69
Myanmar / Burma
58
Eastern Europe
55
Sierra Leone
51
Germany
42
Africa
38
Syria
33
Zambia
32
Liberia
31
India
30
Kenya
30
Ethiopia
22
Bangladesh
22
Eastern Europe and Central Asia
22
Nigeria
19
Zimbabwe
18
Malawi
18
Uganda
17
West and Central Africa
17
Russia
17
Congo, Democratic Republic of
16
Ghana
16
South Africa
16
Latin America and the Carribbean
16
Venezuela
16
Guinea
15
South Sudan
14
Nepal
13
Rwanda
13
Moldova
13
Philippines
11
Lesotho
11
Yemen
11
East and Southern Africa
11
Middle East and North Africa
11
Asia
11
Haiti
10
Tanzania
10
Central African Republic
10
Senegal
9
Afghanistan
9
Cambodia
9
Pakistan
8
Namibia
8
Tajikistan
8
Indonesia
7
Mozambique
7
Eswatini/ Swaziland
7
Jordan
6
Greece
6
Albania
6
Romania
6
Vietnam
6
Côte d’Ivoire / Ivory Coast
5
Somalia
5
Lebanon
5
Thailand
5
Botswana
5
South–East Asia Region
5
Poland
5
Laos
5
Burkina Faso
4
Cameroon
4
Iraq
4
Turkey
4
Sudan
4
North Macedonia
4
Colombia
4
Libya
4
Georgia
4
USA
3
Egypt
3
China
3
Benin
3
Brazil
3
Hungary
3
Sri Lanka
3
Western Pacific Region
3
Estonia
3
Turkmenistan
3
Spain
3
Mali
2
Niger
2
Croatia
2
Madagascar
2
Paraguay
2
Italy
2
Armenia
2
Bulgaria
2
Lithuania
2
Slovakia
2
Iran
2
Kazakhstan
2
Jamaica
2
Belarus
2
Guinea-Bissau
1
Ireland
1
Dominican Republic
1
Serbia
1
Cuba
1
Honduras
1
El Salvador
1
Bolivia
1
Mexico
1
Chile
1
Bhutan
1
Palestine
1
Congo-Brazzaville
1
Uzbekistan
1
Kyrgyzstan
1
North America
1
Denmark
1
Mauritius
1
United Kingdom
1
Portugal
1
Israel
1
Latvia
1
Authors & Publishers
Publication Years
Category
Countries
579
Public Health
115
Clinical Guidelines
96
Key Resources
86
Women & Child Health
58
Pharmacy & Technologies
33
Capacity Building
16
Toolboxes
Mental Health
209
COVID-19
170
TB
165
HIV
148
Planetary Health
142
Refugee
131
Conflict
100
Disability
96
AMR
96
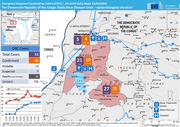
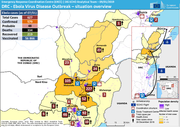
Ebola
69
Rapid Response
62
Global Health Education
53
Pharmacy
51
Health Financing Toolbox
44
NCDs
41
Natural Hazards
29
Caregiver
28
Specific Hazards
21
NTDs
21
Zika
10
Social Ethics
10
Polio
8
Cholera
2
Typhoon
2
Health Financing
1
ECDC Technical Report
In line with ECDC’s recommendations provided in the ’Risk Assessment of HTLV-1/2 transmission by tissue/cell transplantation’ dated 14 March 2012, this Directive replaces the term ‘incidence’ with ‘prevalence’ in the description of endemic areas of HTLV-1/2 i...
In line with ECDC’s recommendations provided in the ’Risk Assessment of HTLV-1/2 transmission by tissue/cell transplantation’ dated 14 March 2012, this Directive replaces the term ‘incidence’ with ‘prevalence’ in the description of endemic areas of HTLV-1/2 i...
Perspectives on Drugs
Infection prevention and control (IPC) practices are of critical importance in protecting the function of healthcare services at all levels and mitigating the impact on vulnerable populations. Although the management of possible COVID-19 cases is usually guided by national policies for specific heal...
It highlights how proven digital innovation can be replicated to curb the spread of COVID-19 in Africa. It also estimates investment required to implement such high impact solutions.
14 June 2022.
The aim of this document is to provide concise advice to public health authorities and guide their prevention, awareness-raising and behaviour change interventions before, during and after upcoming summer events.
факты о легких и туберкулезе - информационная брошюра ; первая часть
(Туберкулез с множественной лекарственной устойчивостью ; вторая часть)
Accessed on September 2019
Interim Assessement Report
The EMA review was started by the Agency’s Committee for Medicinal Products for Human Use (CHMP) to support decision-making by health authorities. This first interim report includes information on seven experimental medicines intended for the treatment of people infecte...
A tutorial for healthcare professionals
Strengthening health-system emergency preparedness.
Infographic/Map on Cholera and Diphteria cases in Yemen
An update from the EU Early Warning System