Filter
4416
Featured
418
1295
Language
4271
80
74
49
32
27
19
11
9
5
5
5
4
4
4
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
Document type
2752
597
560
197
135
75
47
21
12
10
6
3
1
Countries
304
171
120
115
114
107
101
100
97
80
78
77
77
69
66
62
59
59
56
53
45
40
35
34
33
31
31
30
30
30
27
25
25
24
21
20
20
19
17
16
16
15
13
13
13
12
12
12
10
9
9
8
8
8
8
8
7
7
7
7
7
6
6
6
6
6
6
5
5
5
5
5
5
4
4
4
4
4
4
4
4
4
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
511
298
209
160
136
106
88
85
72
33
33
30
28
28
25
24
23
23
21
21
20
19
19
19
18
17
17
16
16
16
16
16
16
15
15
14
14
14
14
13
13
13
13
11
11
11
11
11
11
10
10
10
10
10
10
10
9
9
9
9
9
9
9
9
9
9
8
8
8
8
8
8
8
8
8
8
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
6
6
6
6
6
6
6
6
6
6
6
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Publication Years
854
2955
564
41
2
Category
1886
423
225
223
99
80
50
2
1
Toolboxes
491
338
307
201
185
181
120
92
88
79
75
71
56
55
54
47
33
33
16
12
12
10
9
1
1
For the Fiscal Year 2014-2015, the Health Sector continued to implement interventions and strategies meant to improve the availability, accessibility and utilization of quality healthcare services across public and private health facilities; and to ensure the reduction of the burden of communicable
...
and non-communicable diseases in Rwanda. This annual report highlights key achievements registered by the health sector during the Fiscal Year 2014-2015. Achievements are highlighted under three big components: Health Programs, Health Systems Support and Budget Execution.
more
Save the Children in collaboration with the Bhubaneswar Municipal Corporation (BMC) and the state National Health Mission (NHM) undertook this study in the urban slums of Bhubaneswar city to generate learnings for designing a city-specific public health approach to improve MNH services for the urban
...
poor.
more
The Road Map outlines various strategies which will guide policy makers, development partners, training institutions and service providers in supporting Government efforts towards the attainment of MDGs related to maternal and neonatal health.
Globally, in low-income countries, the average newborn mortality rate is 27 deaths per 1,000 births, the report says. In high-income countries, that rate is 3 deaths per 1,000. Newborns from the riskiest places to give birth are up to 50 times more likely to die than those from the safest places.
... The report also notes that 8 of the 10 most dangerous places to be born are in sub-Saharan Africa, where pregnant women are much less likely to receive assistance during delivery due to poverty, conflict and weak institutions. If every country brought its newborn mortality rate down to the high-income average by 2030, 16 million lives could be saved.
More than 80 per cent of newborn deaths are due to prematurity, complications during birth or infections such as pneumonia and sepsis, the report says. These deaths can be prevented with access to well-trained midwives, along with proven solutions like clean water, disinfectants, breastfeeding within the first hour, skin-to-skin contact and good nutrition. more
... The report also notes that 8 of the 10 most dangerous places to be born are in sub-Saharan Africa, where pregnant women are much less likely to receive assistance during delivery due to poverty, conflict and weak institutions. If every country brought its newborn mortality rate down to the high-income average by 2030, 16 million lives could be saved.
More than 80 per cent of newborn deaths are due to prematurity, complications during birth or infections such as pneumonia and sepsis, the report says. These deaths can be prevented with access to well-trained midwives, along with proven solutions like clean water, disinfectants, breastfeeding within the first hour, skin-to-skin contact and good nutrition. more
A guidance document in simple language for health personnel, setting out their rights and responsibilities in conflict and other situations of violence. It explains how responsibilities and rights for health personnel can be derived from international humanitarian law, human rights law and medical e
...
thics.The document gives practical guidance on:
- The protection of health personnel, the sick and the wounded; - Standards of practice; - The health needs of particularly vulnerable people; - Health records and transmission of medical records; - "Imported" health care (including military health care);
- Data gathering and health personnel as witnesses to violations of international law; - Working with the media
more
National Tuberculosis Programme
The National Strategic Plan (NSP) for Tuberculosis (TB) 2016-2020 builds on the past experiences for the National Tuberculosis Programme and its partners. This NSP provides a roadmap for delivering quality TB prevention and care service to the entire population, ... as an integral part of the country's move toward Universal Health Coverage. Between 1990 and 2015, Myanmar reduced the prevalence of TB by 50%, meeting the targets set by the Millennium Development Goals. Going forward, the country aims to further accelerate the rate decline. more
The National Strategic Plan (NSP) for Tuberculosis (TB) 2016-2020 builds on the past experiences for the National Tuberculosis Programme and its partners. This NSP provides a roadmap for delivering quality TB prevention and care service to the entire population, ... as an integral part of the country's move toward Universal Health Coverage. Between 1990 and 2015, Myanmar reduced the prevalence of TB by 50%, meeting the targets set by the Millennium Development Goals. Going forward, the country aims to further accelerate the rate decline. more
National Strategic Plan for Newborn and Child Health Development (2015-2018)
The Republic of the Union of Myanmar, Ministry of Health, Department of Health, Child Health Division
World Health Organization (WHO), Country Office for Myanmar
(2015)
C_WHO
No publication year indicated
The specific objectives of the plan are to:
- Scale up evidence-based, cost effective interventions through effective strategies within a HSS approach and provide equitable coverage with quality.
- Reduce neonatal mortality by improved home-based newborn ... care, early identification of sick newborns and improved access to institutional newborn care of adequate quality.
- Reduce common childhood illness related mortality (due to pneumonia and diarrhoea in all areas and malaria in endemic areas) by improving key family and community practices, community-based early diagnosis and management and referral care for complicated cases. more
The specific objectives of the plan are to:
- Scale up evidence-based, cost effective interventions through effective strategies within a HSS approach and provide equitable coverage with quality.
- Reduce neonatal mortality by improved home-based newborn ... care, early identification of sick newborns and improved access to institutional newborn care of adequate quality.
- Reduce common childhood illness related mortality (due to pneumonia and diarrhoea in all areas and malaria in endemic areas) by improving key family and community practices, community-based early diagnosis and management and referral care for complicated cases. more
Atlas: country resources for neurological disorders 2004: results of a collaborative study of the World Health Organization and the World Federation of Neurology
recommended
World Health Organization; World Federation of Neurology
(2004)
C_WHO
NEUROLOGY ATLAS presents for the first time, the most
comprehensive collection and compilation of information on
neurological resources across 109 countries. The results confirm
that the available resources including services for neurological
disorders are markedly insufficient; in addition, the
...
re are large
inequities across regions and income groups of countries.
Urgent action is required to enhance the resources available
to address the increasing burden of neurological disorders.
more
The Member States of the Pan American Health Organization/World Health Organization (PAHO/WHO)
that appear in the tables below have used the assessment instrument for mental health systems (WHOAIMS)
(1), as have Anguilla, the British Virgin Islands, Montserrat, and Turks and Caicos, all British
O
...
verseas Territories. For the purpose of this report, the countries and territories were grouped into three subregions, as follows:
Central America, Mexico, and the Latin Caribbean, the non-Latin Caribbean, and South America. The tables
also indicate the year each national WHO-AIMS report was published.
more
Strengthening supply chains to meet the growing demand for family planning will require systems diagnostics, supply chain redesign or adjustments, strategically located storage and distribution systems, adequate staffing and training, and better information about inventory and financing.
Handbook on HIV and Human Rights for National Human Rights Institutions
UNAIDS; Joint United Nations Programme on HIVAIDS and Office of the United Nations High Commissioner for Human Rights
(2007)
C2
Monitoring is a crucial element in any successful programme. It is important to
know if health care facilities – and ultimately countries – are meeting the agreed
goals and objectives for preventing and managing cardiovascular diseases (CVD).
Monitoring is the on-going collection, management
...
and use of information to
assess whether an activity or programme is proceeding according to plan and/
or achieving defined targets. Not all outcomes of interest can be monitored. Clear
outcomes must be identified that relate to the most important changes expected to result from the project and to what is realistic and measurable within the timescale of the project. Once these outcomes have been articulated, indicators can be chosen that best measure whether the desired outcomes are being met.
To allow progress to be monitored, this module provides a set of indicators on
CVD management. Agreeing on a set of indicators allows countries to compare
progress in CVD management and treatment across different districts or
subnational jurisdictions, as well as at a facility level, identify where performance
can be improved, and track trends in implementation over time. Monitoring
these indicators also helps identify problems that may be encountered so that
implementation efforts can be redirected.
This module starts from the collection of data at facility level, which is then
“transferred up” the system: facility-level data are aggregated at subnational level
to produce reports that allow tracking of facility and subnational performance over time and allow for comparison among facilities. National-level data are obtained through population-based surveys.
Implementing a monitoring system requires action at many levels. At national and
subnational levels, staff can determine how best to integrate data elements into
existing data collection systems – such as the routine service-delivery data that are collected through facility-level Health Management Information Systems (HMIS).
In the facility setting, personnel must be aware of what data are needed. Sample
data-collection tools are included, recognizing that countries use different datamanagement systems for HMIS, so the CVD monitoring tools will be adapted to work with the HMIS system being used by the country, such that the indicators can be collected with minimal disruption/work to existing systems and tools
more
Journal of Infection and Public Health 12 (2019) 213–223
Antiretroviral Treatment in the Spotlight: A Public Health Analysis in Latin America and the Caribbean 2013
M. A. Jiménez; S. Cabrera; J. J. Fiol; et al.
Pan American Health Organization; World Health Organization (Americas)
(2013)
C_WHO
Washington, DC, 2013
As a public good, antimicrobial medicines require rational use if their effectiveness is to be preserved. However, up to 50% of antibiotic use is inappropriate, adding considerable costs to patient care, and increasing morbidity and mortality. In addition, there is compelling evidence that antimicro
...
bial resistance is driven by the volume of antimicrobial agents used. High rates of antimicrobial resistance to common treatments are currently reported all over the world, both in health care settings and in the community. For over two decades, the Region of the Americas has been a pioneer in confronting antimicrobial resistance from a public health perspective. However, those efforts need to be stepped up if we are to have an impact on antimicrobial resistance and want to quantify said impact.
more
The most significant finding of the case study for integrating antimicrobial resistance (AMR)into existing programs and mobilising resources for funding in Nigeria, is that most of the AMR activities within the Nigerian National Action Plan (NAP)canalready be incorporated within exi
...
sting programs of the Federal Ministry of Health (FMOH), Federal Ministry of Agriculture and Rural Development (FMARD) and their agencies or institutes. Certain programs and initiatives already have an AMR element incorporated or could,with little effort,include some additional AMR actions, however much is already being planned and has started with existing federal funding and existing staffing and other resources including development partner support and is being driven by significant political will from the ministries as well as implementation support from the Nigerian Centers for Disease Control as the focal point.
more
This technical brief was developed by the UNFPA Global Ageing Network to complement the UN Department of Economic and Social Affairs' (UN DESA) Issue Brief: Older Persons and COVID-19, which emphasized the humanitarian imperative of addressing older persons' specific needs within preparedness and re
...
sponse to the COVID-19 pandemic.
more
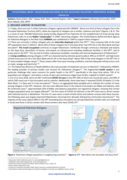
As of 12June 2020, there are 667confirmed COVID-19 casesin the OPT (565 of which are recovered cases), and 48% of which (320 cases) are in East Jerusalem and its suburbs. Additionally, there have been 5 reported COVID-19 deaths (1 in the West Bank, 1 in Gaza and 3 in East Jerusalem)
The Millennium Development Goals (MDGs) showed
that global commitment and collective action
could significantly reduce the disease burdens of
three deadly communicable diseases: HIV/AIDS,
tuberculosis (TB) and malaria. The MDGs helped
focus efforts on these three deadly diseases
and leveraged
...
disease-specific programmes and
financing, thus achieving significant progress.
more