Filter
473
Text search:
Emergency
Use
Authorization
Featured
49
111
Language
Document type
214
99
79
24
22
21
9
3
2
Countries / Regions
18
18
15
13
11
10
9
8
8
8
8
8
7
7
6
6
6
6
6
5
5
5
4
4
4
4
4
4
4
4
4
4
3
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
Publication Years
Category
173
32
23
17
17
11
3
1
Toolboxes
125
27
23
20
19
14
13
10
9
9
9
8
7
6
6
5
4
2
2
2
1
1
1
The preparedness strengthening team deployed to Ghana focused on specific objectives in order to assist the country in becoming as operationally prepared as possible to detect, investigate and report potential EVD cases effectively and safely and to mount an effective response to prevent a larger o
...
COVID-19 Vaccines: 1 Safety Surveillance 2 Manual
While there is no indication that pregnant women have an increased susceptibility to infection with SARS-CoV-2, there is evidence that pregnancy may increase the risk of severe illness and mortality from COVID-19 disease in comparison with non-pregn
...
This document provides answers to FAQs healthcare providers and staff may have about COVID-19 vaccines
an approach to optimize the global impact of COVID-19 vaccines, based on public health goals, global and national equity, and vaccine access and coverage scenarios, first issued 20 October 2020, updated: 13 November 2020, updated: 16 July 2021, latest update: 21 January 2022
Available in English, F
...
The WHO Pharmaceuticals Newsletter provides you with the latest information on the safety of medicinal products and regulatory actions taken by authorities around the world.
In addition, this edition includes summary and recommendations from the virtual meeting of the members of the WHO Programme f
...
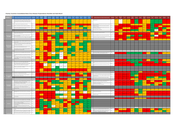
The dashboard is based on assessments made by the International Preparedness Strengthening missions to 14 priority countries against each of the activities outlined in the WHO EVD Checklist at the time of each mission. Updates indicating progress against each of the indicators will be added on an o
...
This Teacher’s Guide accompanies the WHO publication Management of wastes from health-care activities . It provides teaching materials and recommendations for a three day training course, designed mainly for managers of health-care establishments, public health professionals and policy makers
For practitioners in humanitarian and development contexts
These guidelines are applicable to all biomedical, social and behavioural science research for health conducted in India involving human participants, their biological material and data.
The purpose of such research should be: i. directed towards enhancing knowledge about the human condition while
...
Psycho-Social Rehabilitation and Occupational Integration of Child Survivors of Trafficking and Other Worst Forms of Child Labour |
Guiding Principles and Recommendations
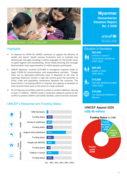
In response to COVID-19, UNICEF continues to support the Ministry of Health and Sports’ Health Literacy Promotion Unit to translate and disseminate messages including in ethnic languages for the border areas on good hygiene and handwashing. Social media boosting and message dissemination reach app
...
While much progress has been achieved over the past year, the Region of the Americas has stubbornly remained the epicenter of the COVID-19 pandemic. PAHO is launching its 2021 COVID-19 Response Strategy and Donor Appeal to continue supporting Latin American and Caribbean countries and territories i
...
Climate change triggers mounting food insecurity, poverty and displacement in Africa