Filter
5905
Featured
591
1471
Language
4891
876
149
127
104
77
45
31
13
9
9
9
8
6
6
6
6
5
5
5
4
4
4
4
4
4
4
4
3
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
Document type
3245
874
852
285
193
175
117
62
31
28
25
10
7
1
Countries
312
194
148
140
131
119
106
103
97
94
92
92
91
86
84
80
75
74
73
72
71
63
58
53
50
46
44
44
43
42
40
39
39
39
38
37
36
35
31
30
28
28
26
25
25
24
23
22
21
20
18
17
16
15
13
13
13
12
11
11
11
11
11
10
10
10
9
9
8
8
8
8
8
8
8
7
7
7
7
6
6
6
5
5
5
5
5
5
5
4
4
4
4
4
3
3
3
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
760
302
280
179
123
123
93
89
88
65
63
59
39
39
39
37
35
33
30
29
29
26
25
25
25
24
24
24
23
22
22
22
22
22
21
20
20
19
19
19
19
19
18
18
18
18
18
18
17
17
16
15
15
15
15
15
14
14
14
13
13
13
13
12
12
12
11
11
11
11
11
11
10
10
10
10
10
10
10
10
9
9
9
9
9
9
9
8
8
8
8
8
8
8
8
8
8
8
8
8
8
8
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
6
6
6
6
6
6
6
6
6
6
6
6
6
6
6
6
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Publication Years
1652
3668
547
36
2
Category
2304
571
300
238
159
142
127
3
2
1
Toolboxes
684
532
452
302
224
201
199
179
158
144
120
114
103
85
79
78
48
40
34
28
25
25
2
1
1
Руководство по самотестированию на ВИЧ и информированию партнеров - Дополнение к сводному руководству по услугам тестирования на ВИЧ
Всемирная организация здравоохранения
Всемирная организация здравоохранения (Европа); European Centre for Disease Prevention and Control (ECDC)
(2018)
C_WHO
ДОПОЛНЕНИЕ
СЛУГИ ТЕСТИРОВАНИЯ НА ВИЧ
ДЕКАБРЬ 2016 г.
Résultats de la Sérologie Réalisée dans le Cadre
de l’Enquête à Indicateurs Multiples
Johnson CC et al. Journal of the International AIDS Society 2017, 20:21594 http://www.jiasociety.org/index.php/jias/article/view/21594 | https://doi.org/10.7448/IAS.20.1.21594
The National Action Plan for Combating Antibiotic-Resistant Bacteria (CARB), 2020-2025, presents
coordinated, strategic actions that the United States Government will take in the next five years to improve the health and wellbeing of all Americans by changing the course of antibiotic resistance.
T
...
his Plan is based on the U.S. Government’s 2014 National Strategy for CARB, and builds on the first National Action Plan released in 2015 by expanding evidence-based activities that have already been shown to reduce antibiotic resistance, such as optimizing the use of antibiotics in human and animal health settings.
more
Le présent plan prend la suite de deux plans nationaux pour préserver l’efficacité des antibiotiques (2001-2005 et 2007-2010), qui visaient à maîtriser et rationaliser la prescription des antibiotiques. Ce troisième plan a pour titre « plan national 2011-2016 d’alerte sur les antibiotique
...
s ». Derrière ce titre se profile une menace de santé publique majeure : un nombre croissant de situations d’impasse thérapeutique contre des infections bactériennes, du fait du développement des résistances aux antibiotiques. Cette menace appelle une mobilisation déterminée et durable de l’ensemble des acteurs impliqués dans le cycle de vie des antibiotiques, afin de concilier des objectifs individuels (qualité de la prise en charge des patients) et collectifs (préservation d’une ressource rare, précieuse et difficile à renouveler).
more
Enquête sur les Indicateurs du Paludisme 2017-2018 (EIPBF) - Burkina Faso
Direction Générale de l’Institut National de la Statistique et de la Démographie (INSD)
Programme National de Lutte contre le Paludisme (PNLP)
(2020)
C2
Accessed on 11.03.2020
Au cours de l’EIPBF 2017-2018, plus de 6 300 ménages ont été enquêtés sur la prévention du paludisme et on a testé plus de 5 500 enfants de 6-59 mois pour le paludisme et l’anémie. Les résultats sont représentatifs pour l’ensemble du pays, pour le milieu urb
...
ains et rural, pour Ouagadougou, et pour les 13 régions.
more
САМОТЕСТИРОВАНИЮ НА ВИЧ И ИНФОРМИРОВАНИЮ ПАРТНЕРОВ
Всемирная организация здравоохранения (ВОЗ)
Всемирная организация здравоохранения (ВОЗ)
(2018)
C_WHO
РУКОВОДСТВО ПО
ДОПОЛНЕНИЕ К СВОДНОМУ РУКОВОДСТВУ ПО УСЛУГАМ ТЕСТИРОВАНИЯ НА ВИЧ
ДЕКАБРЬ 2016 г.
УСЛУГИ ТЕСТИРОВАНИЯ НА ВИЧ
Guide de formation à l’usage des paramédicaux
Le présent guide de formation sur la prise en charge globale des personnes vivant avec le VIH est désormais à la disposition des personnels paramédicaux, des écoles et structures de formation de ces personnels dans la région africaine franco
...
phone. Il a été conçu par des experts africains et français rompus à la formation et engagés depuis de nombreuses années dans la lutte contre le VIH. Il a été testé, amendé et corrigé par un collège de paramédicaux. Il allie clarté, simplicité et intègre l’ensemble des standards internationaux en matière de prise en charge globale, tout en restant proche des réalités concrètes de l’exercice professionnel sur le terrain africain. Il constitue, j’en suis convaincu, un outil de grande qualité au service des professionnels de santé paramédicaux et devrait s’imposer rapidement comme un référentiel francophone incontournable pour la prise en charge globale des personnes vivant avec le VIH.
more
Coronavirus - COVID-19 - Information for travellers
Western Cape Government (health); University of Cape Town; Knowledge Translation Unit (University of Cape Tow Lung Institute)
Western Cape Government (health); University of Cape Town; Knowledge Translation Unit (University of Cape Tow Lung Institute)
(2020)
C2
Updated 24 March 2020
Advisory to start rapid antibody based blood test for COVID-19
Prof. (Dr.) B. Bhargdvd
Government of lndia Department ol Health Research Ministry of Health & Family Welfare & Director-General, ICMR; lndian Councilof Medical Research
(2020)
C2
Dated: 04.04.2020
Putting Human Rights at the Heart of the Response
Topic in Focus: COVID-19 and Women’s Human Rights
15 April 2020
Stay-at-home restrictions and other measures restricting the movement of people contribute to an increase in genderbased violence, a finding confirmed by media reports, official
...
statements and information received from OHCHR field presences and human rights defenders in many countries.
Women and girls already in abusive situations are more exposed to increased control and restrictions by their abusers, with little or no recourse to seek support. Hotlines receive reports of women being threatened with being thrown out of their homes, exposed to the infection, or having financial resources and medical aid withheld.
more
Научная справка
8 апреля 2020 г.
В ответ на растущую пандемию COVID-19 и нехватку ресурсов и реагентов для молекулярного тестирования, многие производители диагностич�
...
�ских тестов разработали и начали продавать простые в использовании экспресс-системы, позволяющие выполнять тестирование вне лабораторий. Эти простые тестовые наборы основаны либо на обнаружении белков вируса COVID-19 в респираторных образцах (например, мокроте, мазке из зева), либо на обнаружении в крови или сыворотке человека антител, образующихся в ответ на инфекцию.
more
COVID Reference 2020.3 DEU
recommended
Lignes directrices provisoires
17 janvier 2020
Le présent document a été rédigé en s’appuyant sur divers documents existants de l’OMS, y compris les lignes directrices de l’OMS relatives au dépistage en laboratoire du MERS-CoV (1-11). À mesure que de nouvelles informations seront r
...
ecueillies concernant l’étiologie, les manifestations cliniques et la transmission de la maladie dans le groupe de patients atteints de maladie respiratoire à Wuhan, l’OMS continuera de suivre l’évolution de la situation et révisera si nécessaire les présentes recommandations.
more
Given the current situation of the COVID-19 pandemic, countries are advised to continue adopting the TB diagnostic algorithms recommended by PAHO / WHO.
Despite the differences in the modes of transmission of TB and COVID-19, certain personal protection measures are relevant for both diseases. Rout
...
ine measures to protect yourself from TB should continue along with additional precautions to protect workers from COVID-19.
more
Laboratory biosafety guidance related to coronavirus disease (COVID-19): Interim guidance, 28 January 2021
recommended
The latest update (28 January 2021) includes the following addition and revision:
biosafety aspects for working with antigen-detecting rapid diagnostic test;
handling new variants of SARS-CoV-2 in the laboratory;
updated assay decontamination before disposal;
personal protectiv
...
e equipment (PPE) for specimen collection;
addressing chemical hazards and their safe disposal; and
the fourth edition of the WHO Laboratory Biosafety Manual (LBM4) is now available and the terminology in this guidance was aligned with the LBM4.
more
SARS-CoV-2 molecular assay evaluation: results
recommended
FIND conducted independent evaluations at the University Hospitals of Geneva (HUG), to verify the limit of detection (LOD) and the clinical performance (as reported by the manufacturers) of the following molecular test kits. The LOD analysis was performed using cultured viral stocks from a clinical
...
isolate from Switzerland, and quantified using an E gene standard. The clinical performance analysis was conducted on extracted samples from individuals suspected to have COVID-19 that were tested using an in-house PCR protocol that was optimized based on the Tib Molbiol assay.
more
he central Sahel region—Burkina Faso, Mali and Niger—is facing a severe humanitarian and protection crisis.
Massive displacement, most of it driven by intense and largely indiscriminate violence perpetrated by a range of armed actors against civilian populations, is taking place across the regi
...
on. While internal displacement is on the rise substantial numbers of refugees have fled to neighboring countries, and the situation risks spilling over into the coastal countries of Benin, Côte d'Ivoire, Ghana, and Togo.
This context is exacerbated by the COVID-19 pandemic, which is already affecting areas hosting refugees and IDPs
more
Over the past weeks, there has been wide media attention on the risk of transmission of the novel coronavirus [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] by asymptomatic individuals. This situation has also been extensively discussed on various platforms globally. The purpose of t
...
his position statement is for the Africa Centres for Diseases Control and Prevention (Africa CDC) to clarify the situation of transmission of SARS-CoV-2 by pre-asymptomatic and asymptomatic Individuals.
more
document d’information scientifique, 17 juin 2020
22 July 2022. This document summarizes current WHO guidance for public health surveillance of coronavirus disease 2019 (COVID-19) in humans caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Available in Arabic, Chinese, English, French, Portuguese and Spanish
https://apps.who.int/iris/handle/10665/334254
COVID-19 has resulted in an unprecedented global crisis. As the pandemic spreads and countries around the world continue to struggle to contain its health and socio-economic consequences, UNRWA is issuing a new humanitarian appeal from August through December 2020 to address the worst impacts of the
...
pandemic on Palestine refugees across the Agency’s five fields of operation. Through this appeal the Agency seeks US$ 94.6 million. The funds requested in this appeal are additional to the previous UNRWA COVID-19 appeal for March to July.
more
Усиление мер реагирования системздравоохранения на COVID-19: техническое руководство № 3
recommended
Усиление мер реагирования системздравоохранения на COVID-19: техническое руководство № 3: поставки основныхлекарственных препаратов и медицинских приборов, 6 апреля
...
2020 г
Strengthening the health systems response to COVID-19: technical guidance #3: supply of essential medicines and health technologies, 6 April 2020
more
What measures can we take to overcome the corona crisis, limit its consequences or use scarce resources efficiently? Every day we experience uncertainties and contradictions on these questions among scientists, health experts, politicians and in society. We must all strive for a broad consensus to o
...
vercome the global COVID-19 pandemic. With our publications IM FOKUS we want to stimulate discussion and promote opinion-forming: We write based on our experience of HIV work. We are not interested in COVID-19 to be equated with HIV, but to discuss which experiences from HIV work could be helpful in dealing with COVID-19. We do not intend to replace scientific papers, nor can we present the current state of knowledge comprehensively and conclusively.
more
Этот документ был подготовлен в рамках инициативы Международного партнерства по правам
человека (IPHR) и его партнеров из Центральной Азии по мониторингу и докумен�
...
�ированию степени
влияния действий правительства в ответ на пандемию COVID-19 в этом регионе. Основное внимание
в этой инициативе уделяется защите основных свобод, таких как свобода выражения мнений, свобода
объединения и свобода собраний; прав на свободу, безопасность и доступ к правосудию; а также прав
уязвимых групп. (the impact of COVID-19 on human rights in kazakhstan )
more
Vaccines (shots) are one of the tools we have to fight the COVID-19 pandemic. Vaccines work with your body’s natural defenses so your body will be ready to fight the virus.
Alternative Languages: Arabic | Spanish | Korean | Russian | Simplified Chinese | Tagalog | Traditional Chinese | Vietnamese
...
https://www.cdc.gov/coronavirus/2019-ncov/communication/print-resources.html?Sort=Date%3A%3Adesc
more
Formation d'orientation pendant 2 jours25 Juin 2020
Published:February 02, 2021DOI:https://doi.org/10.1016/S0140-6736(21)00234-8
The World Health Organization invites clinicians and patients to collect information on COVID-19 in a systematic way and contribute clinical data to the WHO Clinical Platform to expand our knowledge on Post-COVID-19 condition, and support patient care and public health interventions.
WHO’s Post
...
COVID case report form (CRF) has been designed to report standardized clinical data from individuals after hospital discharge or after the acute illness to examine the medium- and long-term consequences of COVID-19. The forms will be available in multiple languages.
more
9 February 2021
22. Februar 2021
Das Epidemiologische Bulletin 8/2021 beschreibt die Chancen, aber auch Risiken und Limitationen bei der Eigenanwendung von Antigen-Selbsttests zum Nachweis einer akuten Infektion mit SARS-CoV-2.
Malaria deaths, Malaria cases
28 Jan. 2021. L’objet de ce document est de fournir des orientations provisoires sur la sécurité biologique en laboratoire applicable à l’analyse d’échantillons cliniques issus de patients.
Ce document fournit des conseils provisoires sur la prévention, l'identification et la gestion de l'infection des travailleurs de la santé dans le contexte de COVID-19. Il s'adresse aux services de santé au travail, aux services ou points focaux de prévention et de contrôle des infections, aux
...
administrateurs des établissements de santé et aux autorités de santé publique, tant au niveau national qu'au niveau des établissements.
more
Orientations provisoires 16 décembre 2020
Le présent document résume les orientations de santé publique actuelles de l’OMS pour la surveillance de la maladie à coronavirus 2019 (COVID-19) chez l’homme due à une infection par le coronavirus 2 du syndrome respiratoire aigu sévère (SARS-Co
...
V-2) (ci-après désignée « surveillance de la COVID-19 »).
more
National Essential Diagnostic List
recommended
WCO Syria Bi-Weekly Situation Report
recommended
Find here the latest information
12 February 2021
Orientations provisoires 19 juillet 2021
Cet article fait partie d’une série d’explications à propos de la mise au point et de la distribution des vaccins.
Available in English, French, Spanish, Arabic, Chinese and Russian
Cet article fait partie d’une série d’explications à propos de la mise au point et de la distribution des vaccins.
Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up
MEDBOX Issue Brief no.18.
The MEDBOX team has compiled a new issue brief on the impact of COVID-19 on PLHIV HIV prevention programmes and treatment for this year's World AIDS Day on 1 December 2021.
The COVID-19 pandemic has impacted the world and consequently increased MHPSS needs across various contexts. While National Societies respond to the rising mental health and psychosocial support needs, they are also adapting to and implementing remote support, such as telephone hotlines or other onl
...
ine services. Accordingly, many trainings in psychological first aid (PFA) of staff and volunteers have moved to online platforms.
Throughout the pandemic, the PS Centre developed online approaches, guidances, adaptable tools, videos, podcasts, and other materials on MHPSS. This was to ensure easy access to tools and resources that assist National Societies in their training efforts in MHPSS during COVID-19.
more
The International Pharmaceutical Federation (FIP) is the global body representing over 4 million pharmacists and pharmaceutical scientists. We work to meet the world's health care needs. FIP is a non-governmental organisation that has been in official relations with the World Health Organization sin
...
ce 1948.
more
Combatting the rising global threat of AMR through a One Health Approach
Contact tracing and quarantine in the context of COVID-19: interim guidance, 6 July 2022
recommended
Contact tracing for COVID-19 is the process of identifying, assessing, and managing people who have been exposed to someone who has been infected with the SARS-CoV-2 virus, while quarantine is the separation of contacts from other people after exposure to a probable or confirmed case of SARS-CoV-2 i
...
nfection. In the context of growing global population immunity from COVID-19 vaccination and past SARS-CoV-2 infection, WHO recommends that identification, contact, quarantine and follow-up should be prioritized for individuals at high risk who have been in contact with a confirmed or probable case of SARS-CoV-2 infection, rather than targeting all contacts. This updated guidance also introduces shorter recommended quarantine periods, including the ability to further shorten quarantine through the use of testing. National and local health authorities should use risk-based approaches to contact tracing and quarantine that include reviewing and adjusting to their local circumstances and disease epidemiology, population immunity, their health system’s capacities, and risk tolerance.
more
This guidance addresses rationale, risk-based scenarios, practical considerations prior to adoption of the self-testing products, quality assurance, safety and ethical considerations, and data management considerations for COVID-19 self-testing. The Africa CDC recommends the use of rapid antigen sel
...
f-testing within two key scenarios. The first includes testing for case identification within scenarios with a high risk of infection, including symptomatic cases and contacts of a confirmed case. The second scenario involves general screening within scenarios of low or unknown risk exposure allowing for self-care such as before gatherings with at-risk individuals and prior to participation in events involving members of different households. Within these scenarios, a positive test result indicates likelihood of current infection, while a negative test result indicates a lower risk of active infection, though it does not rule out infection altogether. All positive cases should be managed following the national COVID-19 management protocol of Member States.ssur
more
Tuberculosis (TB) is the deadliest infectious disease in most low- and middle-income countries, claiming more than 4,000 lives each day. The unprecedented COVID-19 pandemic has seriously impacted people with pre-existing health conditions. People with TB are usually more vulnerable to other infectio
...
ns, including the novel coronavirus, due to pre-existing lung damage. They are also at higher risk of developing complications from COVID-19.
more
Antibiotics 2022, 11(3), 329; https://doi.org/10.3390/antibiotics11030329.
The authors found that the most-represented antibiotics on the Rwandan market were amoxicillin, co-trimoxazole and cloxacillin. No counterfeit antibiotics were found in this study. However, substandard batches with moderate
...
deviations were found, suggesting that regular quality control of antibiotics is needed in Rwanda.
more
Selbstauskunftbogen TBC.
Übersetzung: Gesundheitsamt Würzburg. Vorlage: LGL München
Selbstauskunftbogen TBC.
Übersetzung: Gesundheitsamt Würzburg. Vorlage: LGL München
Sindrome Inflamatoria multissistemica pediatrica (SIM-P) temporalmente associada a COVID-19
Alves Dias, C.; C. Dias Azevedo, G. Fernandes Maciel da Silva et al.
Revista Cientifica FMC
(2020)
CC
Introdução: A pandemia pela COVID-19 é menos incidente na população pediátrica quando comparada à adulta, sendo apenas 2% dos casos abaixo dos 20 anos. Recentemente, a infecção pelo SARS-CoV-2 em crianças e adolescentes está sendo associada a Síndrome Inflamatória Multissistêmica Pedi�
...
�trica (SIM-P), doença de Kawasaki-like. Objetivo: Relatar a ocorrência de uma das apresentações clínicas da COVID-19 na infância, a SIM-P, ressaltando suas manifestações clínicas, laboratoriais e manejo clínico.
more
Rev Panam Salud Publica 45, 2021 |
La publication des résultats de l’EDSC-V intervient quelques années seulement après l’échéance en 2015 des Objectifs du millénaire pour le développement (OMD), le lancement de l’Agenda 2065 de l’Union Africaine, de l’Agenda 2030 des Nations Unies pour le Développement Durable, et a
...
u moment de la finalisation de la
Stratégie nationale de développement de la deuxième génération (2020-2027) dans le cadre de la Vison 2035.Nul doute qu’ils serviront à établir la situation finale ou la situation de référence pour le suivi évaluation des progrès accomplis dans le cadre de ces agendas nationaux et internationaux.
more
The Ministry of Health (MoH) of Syria officially declared a cholera outbreak on 10 September 2022, with the majority of cases reported from Aleppo, Deir-ez-Zor and Al-Hasakeh governorates. Since the situation report issued on 26 September 2022, the number of confirmed and suspected cases continues t
...
o rise. A total of 13 of 14 governorates are now affected, compared with 10 during the last reporting period.
more
Since the notification of the first two confirmed cases of Vibrio cholerae O1 in the greater Port-au-Prince area on 2 October 2022, to 22 October 2022, the Haitian Ministry of Health (Ministère de la Santé Publique et de la Population, MSPP per its French acronym), reported a total of 2,243 suspec
...
ted cases, including 219 confirmed cases, 1,415 hospitalized suspected cases, and 55 registered deaths.
On 20 October 2022, the Dominican Republic Ministry of Public Health confirmed the first imported case of cholera in the country.
more
Current Tropical Medicine Reports (2018) 5:247–256 https://doi.org/10.1007/s40475-018-0166-2 .Purpose of the Review Buruli ulcer (BU) is a necrotizing and disabling cutaneous disease caused by Mycobacterium ulcerans, one of the skin-related neglected tropical diseases (skin NTDs). This article aim
...
s to review the current knowledge of this disease and challenges ahead.
more
GDF is the largest global provider of quality-assured tuberculosis (TB)
medicines, diagnostics, and laboratory supplies to the public sector.
Since 2001, GDF has facilitated access to high-quality TB care in over 130
countries, providing treatments to over 30 million people with TB and procuring
...
and delivering more than $200 million worth of diagnostic equipment
more
Un(e) infirmier/ière travaille dans le secteur des soins de santé et est en charge de certains types d'actes
médicaux et de soins aux patients. Il/elle exerce ses fonctions en concertation avec un médecin ou les exécute de sa propre initiative si la situation relève de l’urgence vitale. Le
...
domaine professionnel de l’infirmier/ière est large et varié. C’est pourquoi il existe différentes spécialisations. L’infirmier/ière en pédiatrie et néonatologie est responsable des soins et de la prise
en charge des enfants malades âgés de 0 à 15 ans.
more
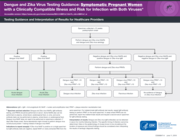
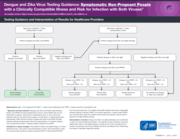
Testing Guidance and Interpretation of Results for Healthcare providers
Testing Guidance and Interpretation of Results for Healthcare Providers Dengue and Zika Virus
Chagas is a parasitic disease that affects over 6 million people in the
world. As the disease typically remains asymptomatic for years, new cases
often go unnoticed and unreported, and most people with the disease
are unaware of their condition. Less than 10% of people affected are
diagnosed and
...
the vast majority do not receive the treatment they need.
If not treated, Chagas may cause irreversible, life-threatening damage to
the heart and other vital organs.
more
At the forefront of DNDi’s efforts to develop new treatments is the need to understand the realities and treatment needs of patients and health care staff in the field. The ultimate goal for human African trypanosomiasis (HAT) is a truly simplified
treatment which can be orally administered, impl
...
emented at the primary health care level, and effective against both stages of the disease.
more
Yaws, a neglected tropical disease (NTD) of the skin caused by the bacterium Treponema pallidum subspecies pertenue, is targeted in the latest WHO NTD Roadmap for eradication by 2030. In January, 2022, WHO published a manual that outlines the key activities that Ministries of Health in endemic count
...
ries should undertake to achieve this goal. The aim of the manual is to provide guidance on surveillance and evaluation of yaws as programmes progress towards eradication. However, yaws eradication in Africa faces several challenges.
more
Le Cadre d’action régional vise à aider les États Membres, l’OMS et tous les autres donateurs et partenaires à travailler ensemble pour remédier systématiquement et progressivement aux diverses faiblesses relevées dans les principaux domaines programmatiques et/ou de contribuer à renforc
...
er les composantes pertinentes des systèmes de santé, de façon à fournir un accès universel et équitable aux
interventions et aux services essentiels liés aux MTN, notamment pour les populations marginalisées et peu accessibles, et à parvenir rapidement à la maîtrise et à l’élimination des MTN.
more
En 2014, la soixante-septième Assemblée Mondiale de la Santé a exprimé sa préoccupation croissante au sujet de la situation
de la RAM, et elle a exhorté les pays membres à renforcer leurs programmes d’action nationale ainsi que la collaboration
internationale. Dans sa résolution WHA67.25
...
l’Organisation Mondiale de la Santé (OMS) a recommandé que soit développé
un Plan d’Action Mondial pour lutter contre la RAM. Ce Plan a été adopté en mai 2015 et recommande notamment la mise en
place d’un système mondial de surveillance de la RAM (GLASS, Global Antimicrobial Résistance Surveillance System). L’objectif
de GLASS est de permettre la collecte, l’analyse et l’échange avec les pays de données standardisées, validées, comparables
sur la résistance aux antimicrobiens.
more
The Coronavirus Disease 2019 (COVID-19) has had a continuous and robust impact on world health. The resulting COVID-19 pandemic has had a devastating physical, mental and fiscal impact on the millions of people living with noncommunicable diseases (NCDs), as they have a higher risk of severe illness
...
and death from COVID-19. COVID-19 has been associated with an
excess in all-cause and cardiovascular disease (CVD) mortality beyond that related to the infection itself and its immediate consequences. Studies in the
United Kingdom (UK) and United States of America (USA) have clearly shown increasing deaths from ischemic heart disease, stroke and hypertensive disease due to COVID-19. Overall, the impact has been greater in individuals with lower socioeconomic status, even in high income nations.
more
The document "Management of Type 2 Diabetes Mellitus" provides comprehensive guidelines for the diagnosis, prevention, and treatment of type 2 diabetes in adults. It emphasizes the importance of individualized glycemic targets, lifestyle interventions like diet and exercise, and the use of medicatio
...
ns such as metformin, SGLT2 inhibitors, and GLP1 receptor agonists to manage blood sugar levels and reduce long-term complications. The document also discusses the screening and management of comorbidities such as hypertension, hyperlipidemia, and diabetic complications like retinopathy, neuropathy, and nephropathy. It highlights the role of diabetes self-management education and support in improving adherence to treatment and patient outcomes. The guidelines are evidence-based and aim to reduce morbidity and mortality associated with type 2 diabetes.
more
The pamphlet "ADHD Medications" provides an overview of medications commonly used to treat ADHD, such as Adderall, Vyvanse, and Ritalin. It explains that these medications do not enhance intelligence and affect individuals differently based on dosage and medical history. Potential dangers of misuse
...
include insomnia, appetite changes, increased heart rate, and reliance on the drugs for studying. Combining ADHD medications with alcohol or sleeping pills is particularly dangerous. The pamphlet emphasizes the importance of proper medical supervision and provides resources for addiction support.
more
Diabetes in pregnancy
National Institute for Health and Care Excellence (NICE)
National Institute for Health and Care Excellence (NICE)
(2016)
CC2
The document "Diabetes in Pregnancy" by NICE (National Institute for Health and Care Excellence) outlines quality standards for managing diabetes in women during pregnancy, with a focus on five key areas. First, it emphasizes the importance of preconception planning for women of childbearing age wit
...
h diabetes. These women should receive guidance on optimizing their health before pregnancy, including achieving target HbA1c levels and taking high-dose folic acid to minimize risks. Second, joint diabetes and antenatal care is recommended for pregnant women with pre-existing diabetes, who should be seen early in pregnancy (ideally by 10 weeks gestation) by a combined diabetes and antenatal team to ensure optimal care throughout their pregnancy.
The third focus area is continuous glucose monitoring (CGM), which should be offered to pregnant women with type 1 diabetes. This includes either real-time CGM or flash monitoring to help improve blood glucose control and reduce complications during pregnancy. Fourth, postnatal testing and referral are essential for women diagnosed with gestational diabetes, who should receive glucose testing after birth to detect any persistent diabetes. Those eligible are referred to the National Diabetes Prevention Programme to lower their risk of developing type 2 diabetes. Lastly, the document recommends annual HbA1c testing for women with a history of gestational diabetes to monitor for type 2 diabetes development.
These standards aim to improve pregnancy outcomes for women with diabetes by providing individualized, accessible, and culturally appropriate care.
more
22 September 2024
HIV testing programmes need to ensure that all clients who test for HIV are provided with correct diagnoses. The accuracy of HIV testing is critical to prevent misdiagnosis, as the consequences of giving an incorrect test result can be serious for clients, HIV testing services, HIV programmes and pu
...
blic health.
With the evolution of global HIV epidemiology, HIV testing approaches must also evolve to maintain accuracy and efficiency in population-level diagnosis. Reports suggest that misdiagnosis of HIV status may occur when suboptimal testing algorithms and out-of-date testing strategies are used. As a result of changing epidemiology and declining HIV positivity in testing, WHO recommends all countries use a standard three-test strategy to ensure a PPV of at least 99%, minimizing false-positive misdiagnosis. The WHO-recommended HIV testing strategy, along with quality assurance measures such as retesting to verify a positive diagnosis prior to initiation of HIV treatment, is cost-effective as it prevents misdiagnosis and unnecessary initiation of costly lifelong treatment.
This implementation guide provides practical advice on switching to a three-test strategy and instituting other measures that can help national HIV programmes deliver high-quality, accurate HIV testing services and ensure that misdiagnosis is minimized.
more
Developing protocols for use with refugees
and internally displaced persons
2nd edition
• provide scientific information on the safety, efficacy, and quality control/ quality assurance of widely used medicinal plants, in order to facilitate their appropriate use in Member States;
• provide models to assist Member States in developing their own mono- graphs or formularies for these
...
or other herbal medicines; and
• facilitate information exchange among Member States.
more