Filter
766
Text search:
specimen
transport
Featured
136
253
Language
Document type
475
155
45
44
27
7
7
4
1
1
Countries / Regions
60
34
29
29
28
25
20
20
17
15
14
14
13
13
11
9
9
9
9
9
8
7
7
6
6
5
5
5
5
4
4
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
Publication Years
Category
327
99
48
31
14
12
11
Toolboxes
102
99
85
62
36
21
16
15
9
9
9
8
7
4
4
4
4
2
2
2
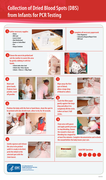
This clinical job aid provides health care workers with information on how to collect specimens for early infant diagnosis on dried blood spots, as well as drying and packaging for transport.
RBC/IHDPC/ EID Division | November2011 - The aim of the standard operating procedures is to guide health care providers and public health
experts from various levels of the health system in the implementation of enhanced surveillance of meningococcal meningitis.
The objective of this guidance document is to support the public health professionals
in implementing effective surveillance of cholera in at-risk, endemic and epidemic
areas. This document has been developed by the Surveillance Working Group of the
Global Task Force for Cholera Control (GTFCC) a
...
Due to the anticipated significant rise in VL testing occasioned by Ghana’s adaptation of 2016 ART guidelines, it has become necessary to develop this VL scale-up and operational plan to assure complete client access to laboratory monitoring towards the achievement of the third 90 of the HIV care
...
WHO TRS N°1012.
Key updates include: (i) surveillance strategies, including cross-sectoral linking of systems and suitable diagnostics; (ii) the latest recommendations on human and animal immunization; (iii) palliative care in lowresource settings; (iv) risk assessment to guide management of bite
...
The main objectives of these guidelines are to:
1. contribute to the quality assurance of medicinal plant materials used as the source for herbal medicines to improve the quality, safety and efficacy of finished herbal products; 2. guide the formulation of national and/or regional GACP guideli ...
1. contribute to the quality assurance of medicinal plant materials used as the source for herbal medicines to improve the quality, safety and efficacy of finished herbal products; 2. guide the formulation of national and/or regional GACP guideli ...
This is a case-ascertained prospective investigation of all identified health care contacts working in a health care facility in which a laboratory confirmed 2019-nCoV infected patient (see 2.2 Study population) receives care. Note that this study can be done in health care facilities at all 3 level
...
Nested case-control study of health workers exposed to confirmed COVID-19 patients.
Similar objectives to the cohort study but case-control studies may be cheaper and provide robust evidence to characterize and assess the risk factors for SARS-CoV-2 infection in health workers exposed to COVID-19 p
...
Interim Guidance 31 march 2020
WHO has established a shipment mechanism to expedite and cover the costs of the shipment of clinical samples from patients with suspected COVID-19 from the country of collection to one of the WHO reference laboratories providing confirmatory molecular testing for COVI
...
Policy framework.
The following protocol has been designed to investigate the extent of infection, as determined by seropositivity in the general population, in any country in which COVID-19 virus infection has been reported. Each country may need to tailor some aspects of this protocol to align with public health, l
...
The purpose of this document is to provide interim guidance to laboratories and stakeholders involved in laboratory testing of patients who meet the definition of suspected case of pneumonia associated with a novel coronavirus identified in Wuhan, China.
19 March 2020
Laboratory biosafety guidance related to coronavirus disease (COVID-19): Interim guidance, 28 January 2021
recommended
The latest update (28 January 2021) includes the following addition and revision:
biosafety aspects for working with antigen-detecting rapid diagnostic test;
handling new variants of SARS-CoV-2 in the laboratory;
updated assay decontamination before disposal;
personal protectiv
...
The National Guidelines for HIV-1 Viral Load Laboratory Testing support plans to scale up viral load (VL) testing to reach the 90-90-90 targets in India. This phased scale-up includes the setup of 70 additional VL testing laboratories nationally. These guidelines include laboratory design considerat
...
Stop TB`s GDF provides a wide range of diagnostic equipment and laboratory supplies in its Diagnostics Catalog