Filter
6073
Filtered Results: 6073
Text search:
sample
Featured
Recommendations
511
New Publications
1718
Language
Document type
No document type
3716
Studies & Reports
930
Guidelines
710
Manuals
271
Strategic & Response Plan
146
Training Material
113
Fact sheets
97
Situation Updates
52
Resource Platforms
14
Infographics
14
Brochures
4
Videos
4
Online Courses
2
Countries / Regions
India
361
Kenya
192
Global
186
Nigeria
156
Ethiopia
145
Sierra Leone
143
Nepal
139
Liberia
135
South Africa
130
Uganda
121
Rwanda
111
Malawi
109
Western and Central Europe
104
Myanmar / Burma
102
Tanzania
99
Bangladesh
95
Ghana
86
Zambia
86
Latin America and the Carribbean
75
Congo, Democratic Republic of
71
Africa
67
Syria
58
Zimbabwe
54
Philippines
53
Namibia
48
Cambodia
46
Lesotho
46
West and Central Africa
43
Ukraine
42
Eastern Europe
39
East and Southern Africa
38
Senegal
36
Indonesia
35
Guinea
34
Haiti
34
Burkina Faso
33
South Sudan
32
Pakistan
30
Mozambique
30
Asia
30
Russia
30
Brazil
29
South–East Asia Region
28
Mali
27
Cameroon
27
Botswana
26
Afghanistan
24
Germany
23
Middle East and North Africa
20
Venezuela
20
Jordan
19
Lebanon
19
Eswatini/ Swaziland
19
Colombia
19
Benin
17
Yemen
16
USA
14
Eastern Europe and Central Asia
14
Madagascar
13
Tajikistan
13
Sudan
12
Côte d’Ivoire / Ivory Coast
11
Niger
11
Somalia
11
Iraq
10
Thailand
10
China
10
Peru
10
Vietnam
10
Central African Republic
9
Angola
9
Western Pacific Region
9
Libya
8
Albania
8
Georgia
8
Burundi
7
Sri Lanka
7
Moldova
7
Egypt
6
Chad
6
Papua New Guinea
6
Argentina
6
Ecuador
6
Chile
6
Palestine
6
Laos
6
Iran
6
Kazakhstan
6
Saudi Arabia
5
North Macedonia
5
Bolivia
5
Kyrgyzstan
5
Tunisia
5
United Kingdom
5
Spain
5
Togo
4
Turkey
4
Serbia
4
Guatemala
4
Bhutan
4
Italy
4
Armenia
4
Southern Africa
4
Timor Leste/ East Timor
4
North America
4
Portugal
4
Guinea-Bissau
3
Morocco
3
Malaysia
3
Singapore
3
Dominican Republic
3
Honduras
3
El Salvador
3
Paraguay
3
Poland
3
Canada
3
Qatar
3
Turkmenistan
3
Belarus
3
Bosnia and Herzegovina
3
Gambia
2
Mauritania
2
Ireland
2
Mexico
2
Other region
2
Fiji
2
Mongolia
2
Congo-Brazzaville
2
Estonia
2
Romania
2
Uzbekistan
2
Gabon
2
Jamaica
2
Belgium
2
France
2
Mauritius
2
Azerbaijan
2
Israel
2
Vanuatu
1
Hungary
1
Croatia
1
Cuba
1
Greece
1
Nicaragua
1
North Korea
1
Djibouti
1
Australia
1
Uruguay
1
Japan
1
Denmark
1
Maldives
1
Algeria
1
Authors & Publishers
Publication Years
Category
Countries
2461
Clinical Guidelines
478
Women & Child Health
281
Public Health
280
Key Resources
247
Capacity Building
145
Pharmacy & Technologies
101
Toolboxes
COVID-19
534
Mental Health
458
HIV
429
TB
364
Rapid Response
235
Ebola
222
Disability
180
NTDs
176
AMR
175
Caregiver
138
Planetary Health
133
Conflict
103
Pharmacy
103
NCDs
94
Refugee
86
Zika
59
Polio
58
Global Health Education
54
Cholera
51
Natural Hazards
48
Specific Hazards
40
Health Financing Toolbox
32
Social Ethics
14
Typhoon
2
(2016) ‘Structural Drivers and social protection: mechanisms of HIV risk and HIV prevention for South African adolescents’. JIAS, 2016, 19(1):20646. http://dx.doi.org/10.7448/IAS.19.1.20646.
Social protection and HIV: Research implications for policy - 1 of 6
WHO
Considering programmatic implications of rising levels of HIV drug resistance: finalizing the Global Action Plan
Webinars 12 13 Dec 2016
Your healthcare team has decided you or your loved one has an infection that requires antibiotics, or needs antibiotics to prevent an infection in certain circumstances, such as before surgery - Fact Sheet for Patients
Научная справка
24 апреля 2020 г.
При инфицировании в естественных условиях выработка иммунитета к патогенному микроорганизму проходит в несколько этапов и заним�...
Accessed: 01.05.2020
Breastmilk is the best source of nutrition for infants, including infants whose mothers have confirmed or suspected coronavirus infection. As long as an infected mother takes appropriate precautions—outlined below—she can breastfeed her baby. Breastmilk contains antibodie...
Here you can download training material, public resources, posters, brochures for different audiences and group spaces
New research explores the stress children in World Vision programmes in the Middle East and Eastern Europe region are under due to COVID-19. In addition to their fear that they themselves or their loved ones will catch the disease, children worry about economic hardships, the loss of their education...
Updated 28 December 2020
African Journal of Emergency Medicine
Volume 11, Issue 1, March 2021, Pages 132-139
The Ministry of Health (MoH) of Syria officially declared a cholera outbreak on 10 September 2022, with the majority of cases reported from Aleppo, Deir-ez-Zor and Al-Hasakeh governorates. Since the situation report issued on 26 September 2022, the number of confirmed and suspected cases continues t...
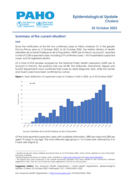
Since the notification of the first two confirmed cases of Vibrio cholerae O1 in the greater Port-au-Prince area on 2 October 2022, to 22 October 2022, the Haitian Ministry of Health (Ministère de la Santé Publique et de la Population, MSPP per its French acronym), reported a total of 2,243 suspec...
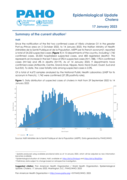
Since the notification of the first two confirmed cases of Vibrio cholerae O1 in the greater Port-au-Prince area on 2 October 2022, to 14 January 2023, the Haitian Ministry of Health (Ministère de la Santé Publique et de la Population, MSPP per its French acronym), reported a total of 24,232 suspe...
Schistosomiasis is an acute and chronic parasitic disease caused by blood flukes (trematode
worms) of the genus Schistosoma. At least 249 million people required preventive treatment in
2012. Preventive treatment, which should be repeated over a number of years, will reduce and
prevent morbidity.
Reproductive health needs are particularly acute in countries affected by armed conflict. Reliable information
on aid investment for reproductive health in these countries is essential for improving the efficiency and effectiveness of
aid. The purpose of this study was to analyse official developm...
On 16 February 2022, Malawi received confirmation of Wild Poliovirus Type 1 (WPV1) from an acute flaccid paralysis (AFP) case in Lilongwe.
The module is currently available in English, French, Nepali, Portuguese, Russian, and Spanish