Filter
6504
Filtered Results: 6504
Text search:
effectiveness
Featured
Recommendations
577
New Publications
1599
Language
Document type
No document type
3617
Studies & Reports
1059
Guidelines
896
Manuals
347
Strategic & Response Plan
248
Fact sheets
158
Training Material
84
Situation Updates
34
Infographics
21
Resource Platforms
19
Brochures
13
Online Courses
4
Videos
2
Dashboards/Maps
1
App
1
Countries / Regions
Global
275
India
220
Kenya
172
Western and Central Europe
141
South Africa
137
Ethiopia
136
Uganda
121
Sierra Leone
112
Nepal
110
Nigeria
105
Liberia
95
Malawi
93
Africa
91
Zambia
87
Myanmar / Burma
83
Latin America and the Carribbean
82
Tanzania
78
Rwanda
76
Ghana
68
Bangladesh
68
Eastern Europe
58
Syria
53
Russia
50
Namibia
49
Congo, Democratic Republic of
48
Philippines
48
Ukraine
46
South–East Asia Region
43
Zimbabwe
42
Asia
42
Lesotho
40
Indonesia
34
East and Southern Africa
33
West and Central Africa
33
Guinea
32
Germany
32
Cambodia
32
Senegal
31
Brazil
30
South Sudan
29
Haiti
28
Mozambique
28
Botswana
28
Middle East and North Africa
28
Eastern Europe and Central Asia
25
Burkina Faso
22
Pakistan
22
Cameroon
20
Eswatini/ Swaziland
17
Colombia
17
Yemen
16
Western Pacific Region
16
USA
14
Vietnam
13
Thailand
12
Sudan
12
Laos
12
Somalia
11
Jordan
11
Afghanistan
11
Benin
11
Lebanon
10
China
10
Peru
10
Albania
10
Central African Republic
9
Tajikistan
9
Madagascar
8
Paraguay
8
Kyrgyzstan
8
Mali
7
Iraq
7
Argentina
7
Burundi
7
Ecuador
7
Sri Lanka
7
Georgia
7
Côte d’Ivoire / Ivory Coast
6
Angola
6
Chile
6
Southern Africa
6
North America
5
Niger
4
Turkey
4
Serbia
4
Venezuela
4
Estonia
4
Iran
4
Kazakhstan
4
Turkmenistan
4
United Kingdom
4
Papua New Guinea
3
North Macedonia
3
Bolivia
3
Libya
3
Palestine
3
Armenia
3
Canada
3
Uzbekistan
3
Bosnia and Herzegovina
3
Morocco
2
Saudi Arabia
2
Togo
2
Egypt
2
Gambia
2
Singapore
2
Honduras
2
El Salvador
2
Mexico
2
Fiji
2
Bhutan
2
Poland
2
Timor Leste/ East Timor
2
Lithuania
2
Moldova
2
Gabon
2
Jamaica
2
Japan
2
Tunisia
2
Portugal
2
Spain
2
Azerbaijan
2
Guinea-Bissau
1
Chad
1
Malaysia
1
Ireland
1
Hungary
1
Croatia
1
Greece
1
Other region
1
Bulgaria
1
Romania
1
Denmark
1
France
1
Maldives
1
Israel
1
Authors & Publishers
Publication Years
Category
Countries
2135
Clinical Guidelines
516
Public Health
421
Women & Child Health
336
Key Resources
326
Capacity Building
108
Pharmacy & Technologies
53
Toolboxes
COVID-19
603
Mental Health
593
HIV
552
TB
354
AMR
232
Planetary Health
212
Disability
206
Rapid Response
203
Caregiver
186
Ebola
158
NTDs
146
NCDs
137
Conflict
134
Pharmacy
117
Refugee
94
Global Health Education
85
Natural Hazards
83
Health Financing Toolbox
77
Cholera
47
Specific Hazards
39
Polio
37
Zika
33
Social Ethics
24
Typhoon
4
Health Financing
3
Programmatic update
April 2012
Executive Summary
Integrated Management of pregnancy and childbirth
Family planning: a global handbook for providers
recommended
4th edition 2022 of the Handbook includes new WHO recommendations that expand contraceptive choices. Also, guidance on starting ongoing contraception following emergency contraception is provided.
Drawing on lessons from recent outbreaks, this new edition details tangible me...
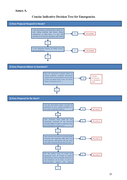
The document contains 2 decision trees, the first short one is to act fast and the second is to design better interventions
Design Parameters and Construction Details
These Guidelines comprise a General Guidelines document that provides the basic parameters of DG ECHO humanitarian health assistance, complemented by specific Technical Guidance in annex.
An Illustrated Guide for Surgeons
Third Edition
Disabled people in developing countries are the poorest of the poor: if we are serious about tackling extreme poverty, our development work has to target them. The post-2015 development framework offers hope that disabled people will finally get the prominence they deserve on the global development ...
This working draft develops guidance on conducting effective evaluations of conflict prevention and peacebuilding work. The current working draft will be used for a one year application phase through 2008. It is the result of an ongoing collaborative project by the OECD DAC Networks on Development E...
A catalogue of family planning job aids for low resource settings, which includes a brief description and key messages as well as pros and cons for each job aid.
Accessed June 2014.
Available in: English, French, Chinese, Spanish, Russian, Arabic, Thai, Korean, Tajik, Vietnamese, Uzbek
http://www.who.int/disabilities/cbr/guidelines/en/
Ebola Response Roadmap
recommended
The World Health Organization is issuing a "roadmap" to guide and coordinate the international response to the outbreak of Ebola virus disease in West Africa.
The aim is to stop ongoing Ebola transmission worldwide within 6–9 months, while rapidly managing the consequences of any further interna...
DG Echo Funding Guidelines