Filter
1385
Featured
136
326
Language
Document type
888
188
170
60
25
24
23
5
1
1
Countries
68
43
38
38
33
30
26
23
21
21
20
20
19
19
19
17
17
15
15
15
14
13
11
9
9
8
8
7
7
7
7
7
6
6
6
5
5
4
4
4
4
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
Publication Years
Category
443
162
76
44
30
22
4
Toolboxes
218
174
157
61
59
43
33
29
27
23
22
21
19
17
17
7
6
3
2
2
2
1
1
MSF Essential Drugs - Practical Guidelines 2022
recommended
Practical guide intended for physicians, pharmacists, nurses and medical auxiliaries. This guide is not a dictionary of pharmacological agents. It is a practical manual intended for health professionals, physicians, pharmacists, nurses and health auxiliaries invoved in curative care and
...
This report provides an update on the key facets of HIV treatment access, including the latest HIV treatment guidelines from World Health Organization (WHO), an overview on pricing for first-line, second-line, and salvage regimens, and a summary of the opportunities for – and threats to – expand
...
These guidelines are based on the 3rd Edition of the WHO Guidelines (Published 2015) World Health Organization’s Guidelines for the treatment of malaria. Additional literature surveys have been undertaken. Factors that were considered in the choice of therapeutic options included effectiveness, sa
...
Nat Commun 9, 5370 (2018). https://doi.org/10.1038/s41467-018-07804-8. Mycobacterium ulcerans is the causative agent of Buruli ulcer, a neglected tropical skin disease that is most commonly found in children from West and Central Africa. Despite the severity of the infection, therapeutic options are
...
The goal of this addendum is to help management and staff
minimize the risk of TB transmission at facilities in resource limited settings in which a.) HIV-infected persons receive diagnosis, care, treatment,
and/or support, and b.) there is a high prevalence of HIV infection, both known
and
...
This publication seeks to describe the best treatments and practices based on the scientific evidence available at the time of writing as evaluated by the authors and may change as a result of new research. Readers need to apply this knowledge to patients in accordance with the guidelines and laws o
...
This publication is intended for professionals training or practicing in mental health and not for the general public. The opinions
expressed are those of the authors and do not necessarily represent the views of the Editor or IACAPAP. This publication seeks to
describe the best treatments and pra
...
The international community sits at the tipping pointof a post-‐antibiotic era, where common bacterial infections are no longer treatable with the antibiotic armamentarium that exists. In South Africa, t
...
The Libyan national action plan has been aligned with WHO five objectives. Analysis of the current situation and addressing the gaps and the needs to reach the main goal “one health” approach involves several national sectors and actors, including human and veterinary health, agriculture and foo
...
BUKO Pharma-Kampagne has investigated the causes and consequences of antibiotic resistance in India, South Africa, Tanzania and Germany. Together with our partners we collected data and did interviews with numerous stakeholders. The outcome is presented in a brochure that is now available in English
...
The internationally recognized criteria for diagnosis of neurocysticercosis include a requirement for neuroimaging techniques, such as computerized tomography (CT) and/or magnetic resonance imaging (MRI), ideally supported by serology. These facilities are not available in all settings, especially i
...
Pathogens . 2021 Nov 16;10(11):1493.doi: 10.3390/pathogens10111493
.Chronic manifestations of Chagas disease present as disabling and life-threatening condi-tions affecting mainly the cardiovascular and gastrointestinal systems. Although meaningful research has outlined the different molecular mech
...
The environment in which young people live, learn and play significantly affects their decisions about whether to consume alcohol. Environmental factors are the main risk factors driving alcohol consumption and related harm among young people. Environments that normalize alcohol consumption – term
...
This integrated operational framework provides an overview of the connections between mental health, neurological and substance use (MNS) conditions, and their links to health, well-being and the broader public health and sustainable development agenda. The need for integrated approaches is increasi
...
The larval stage of the parasite Taenia solium can encyst in the central nervous system causing neurocysticercosis, which is the main cause of acquired epilepsy in the countries in which the parasite is endemic. Endemic areas are those with the presence (or likely presence) of the full life cycle of
...
Since the Alma Ata Declaration in 1978, community health volunteers (CHVs) have been at the forefront, providing health services, especially to underserved communities, in low-income countries. However, consolidation of CHVs position within formal health systems has proved to be complex and continue
...
Third edition
Accessed November 2017
Accessed: 19.10.2019
Клинический протокол для Европейского региона ВОЗ
The reports bring together the latest findings and conclusions about the state of resistance to artemisinins and artemisinin-based combination therapy (ACT), summarize WHO’s current policy and treatment recommendations, and highlight areas of concern.
guidance for health managers, health workers, and activists
ATLAS on substance use (2010)— Resources for the prevention and treatment of substance use disorder
Accessed: 14.03.2019
Nature | Vol 600 | 2 December 2021 |
The key updates include: content update in various sections based on new evidence; design changes for enhanced usability; a streamlined and simplified clinical assessment that includes an algorithm for follow-up; inclusion of two new modules
- Essential Care and Practice that includes general guid
...
The manual is written for clinicians working at the district hospital (first-level referral care) who diagnose and manage sick adolescents and adults in resource constrained settings. It aims to support clinical reasoning, and to provide an effective clinical approach and protocols for the managemen
...
Treatment Recommendations for Adult Inpatients
DRUGS REGULATORY UNIT | NOVEMBER 2009 | SECOND EDITION
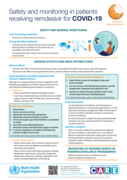
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
This publication is an updated version of the Management of Tuberculosis and HIV Coinfection clinical protocol released in 2007 by the WHO Regional Office for Europe. It is intended for all health care workers involved in preventing, diagnosing, treating and caring for people living with TB and HIV
...
Sixth edition
2007
Sub-Saharan African Journal of Medicine: Year : 2014 | Volume : 1 | Issue : 1 | Page : 1-14
Zambia National Formulary 04
recommended
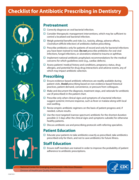
Dentists are uniquely positioned to play a role in pre enting the spread of antibiotic resistance. Here are se en simple “how-tos” for safe, appropriate antibiotic prescribing and use when treating dental infections
Checklist for Antibiotic Prescribing in Dentistry - Fact Sheet
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
Este manual pretende estandarizar la práctica del SFT para que el QF pueda trabajar
con los usuarios de forma consistente y estandarizada a nivel nacional.
This guideline is intended to provide requirements to applicants wishing to submit
applications for registration of medicines in Botswana.
Information for families affected
Research Article
BMC Infectious Diseases 2012, 12:262; doi:10.1186/1471-2334-12-262
A survey of prevention, testing and treatment policies and practices
2nd Generation HIV Surveillance in Pakistan, Round 5
Module: Disorders due to substance abuse mhGAP
recommended
Accessed: 21.03.2019
mhGAP Training Manual for the mhGAP Intervention Guide for mental, neurological and substance use disorders in non-specialized health settings – version 2.0 (for field testing)
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
Tuberculosis. Practical guide for clinicians, nurses, laboratory technicians and medical auxiliaries
This Tuberculosis guide has been developed jointly by Médecins Sans Frontières and Partners In Health. It aims at providing useful information to the clinicians and health staff for the comprehensive management of tuberculosis. Forms of susceptible and resistant tuberculosis, tuberculosis in child
...
Coordinated Use of Anthelminthic Drugs in Control Interventions: a Manual for Health Professionals and Programme Managers
Antimicrobial resistance (AMR) has emerged as a major public health problem all over the world. Infections caused by resistant microbes fail to respond to treatment, resulting in prolonged illness and greater risk of death. This document focuses on the mechanism to develop a practically applicable h
...
Ethiopian Medicines Formulary
recommended
2nd edition
Accessed November, 2017
Document No. : FDA/SMC/SMD/GL-RAR/2013/01
The Zimbabwe National Pharmacovigilance Policy Handbook, 2nd Edition updates the November 2013 version to indicate the Zimbabwe National Pharmacovigilance (PV) Centre’s compliance with the WHO Pharmacovigilance Indicators Handbook 2015.
The END TB Strategy
Интеграция совместного оказания услуг в связи с ТБ и ВИЧ во всеобъемлющий пакет помощи для потребителей инъекционных наркотиков
Accessed November 2017
National Tuberculosis Programme and Senior Paediatricians
This guideline was first developed in 2007 but further updated in 2012 and 2016 to ensure the use of the latest evidence-based international recommendations on childhood TB. The guidelines will fill the gaps in a systematic approach to T ...
This guideline was first developed in 2007 but further updated in 2012 and 2016 to ensure the use of the latest evidence-based international recommendations on childhood TB. The guidelines will fill the gaps in a systematic approach to T ...
Dialogues Clin Neurosci. 2017 Jun; 19(2): 93–107.
Venturini et al. BMC Infectious Diseases 2014, 14(Suppl 1):S5 http://www.biomedcentral.com/1471-2334/14/S1/S5
Version 3.0
Accessed: 24.11.2019
HIV i-Base
July 2018
HIV Treatment
Policy Brief
July 2017
Made under Section 5 (c) of the Tanzania Food, Drugs and Cosmetics Act, 2003 | Second Edition