Filter
409
Text search:
Clinical
characterization
Featured
Recommendations
46
New Publications
71
Language
Document type
No document type
145
Guidelines
101
Studies & Reports
88
Manuals
30
Strategic & Response Plan
22
Fact sheets
17
Situation Updates
4
Training Material
2
Countries / Regions
Latin America and the Carribbean
17
Global
17
India
16
Africa
14
Brazil
11
Kenya
8
Nigeria
6
Uganda
5
South Africa
5
Colombia
5
West and Central Africa
5
Venezuela
5
Germany
4
East and Southern Africa
4
Western Pacific Region
4
Liberia
3
Ghana
3
Philippines
3
Tanzania
3
Mozambique
3
Namibia
3
Angola
3
Middle East and North Africa
3
Russia
3
Burkina Faso
2
Sierra Leone
2
Congo, Democratic Republic of
2
USA
2
Cameroon
2
Zimbabwe
2
Zambia
2
Peru
2
Bolivia
2
Chile
2
Western and Central Europe
2
Tajikistan
2
Guinea-Bissau
1
Ethiopia
1
Haiti
1
Nepal
1
Pakistan
1
Jordan
1
Afghanistan
1
Syria
1
Cambodia
1
Rwanda
1
Indonesia
1
Bangladesh
1
Malawi
1
Benin
1
Argentina
1
Eswatini/ Swaziland
1
Ukraine
1
Ecuador
1
South–East Asia Region
1
Eastern Europe and Central Asia
1
Eastern Europe
1
Vietnam
1
France
1
Portugal
1
Authors & Publishers
Publication Years
Category
Countries
102
Clinical Guidelines
52
Public Health
22
Pharmacy & Technologies
20
Key Resources
9
Women & Child Health
5
Capacity Building
3
Toolboxes
COVID-19
73
Rapid Response
53
AMR
40
NTDs
29
HIV
16
Pharmacy
16
TB
15
Mental Health
12
Ebola
10
Zika
10
Planetary Health
9
NCDs
6
Cholera
5
Disability
4
Natural Hazards
3
Caregiver
3
Polio
2
Conflict
2
Refugee
2
Specific Hazards
2
Global Health Education
1
Health Financing Toolbox
1
This document shall serve as the most comprehensive set of guidelines on the safe management of waste generated from heath care activities in the country. It incorporates the requirements of all Philippine laws and regulations governing HCWM and is designed for the use of individuals, public and pri
...
Nature Medicine, https://doi.org/10.1038/s41591-021-01283-z
More than two years since the first SARS-CoV-2 infections were reported, the COVID-19 pandemic remains an acute global emergency. In this Strategic Preparedness, Readiness and Response plan for 2022, WHO sets out a number of key strategic adjustments that, if implemented rapidly and consistently at
...
2nd edition. The purpose of the WHO human health risk assessment toolkit: chemical hazards is to provide its users with guidance to identify, acquire and use the information needed to assess chemical hazards, exposures and the corresponding health risks in their given health risk assessment contexts
...
This document focus on the direct consequences of the virus (morbidity and mortality) in specific populations and on the results of measures aimed at mitigating the spread of the virus, with indirect impacts on socio-economic conditions. In this complex scenario, the gender approach has not received
...
Palliative care has been shown to provide significant and diverse benefits for patients with serious, complex,or life-limiting health problem.
Jamison DT, Gelband H, Horton S, Jha P, Laxminarayan R, Mock CN, Nugent R., editors. Disease Control Priorities, 3rd Edition,
Volume 9: Improving Health an
...
Waste Management & Research 39(1) DOI: 10.1177/0734242X211029175
Cases of monkeypox (MPX) acquired in the EU have recently been reported in nine EU Member States (Austria, Belgium, France, Germany, Italy, Portugal, Spain, Sweden, and the Netherlands).
Monkeypox (MPX) does not spread easily between people. Human-to-human transmission occurs through close contact
...
Glob Heart . 2020 Oct 13;15(1):69. doi: 10.5334/gh.891.
The goal of the global outbreak response for monkeypox is to stop human-to-human transmission of monkeypox, with a priority focus on communities at high risk of exposure which may differ according to context, and to effectively use strong public health measures to prevent onward spread of the diseas
...
This document aims to provide interim guidance for microbiology and virology experts, other laboratory professionals, laboratory managers, infectious disease programme managers, public health professionals and other stakeholders that provide primary, confirmatory or advanced testing for SARS-CoV-2,
...
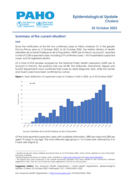
Since the notification of the first two confirmed cases of Vibrio cholerae O1 in the greater Port-au-Prince area on 2 October 2022, to 22 October 2022, the Haitian Ministry of Health (Ministère de la Santé Publique et de la Population, MSPP per its French acronym), reported a total of 2,243 suspec
...
Current Tropical Medicine Reports (2018) 5:247–256 https://doi.org/10.1007/s40475-018-0166-2 .Purpose of the Review Buruli ulcer (BU) is a necrotizing and disabling cutaneous disease caused by Mycobacterium ulcerans, one of the skin-related neglected tropical diseases (skin NTDs). This article aim
...
On 15–16 December 2020, WHO and the Medicines for Malaria Venture co-convened a technical consultation to consider the preferred product characteristics (PPCs) for drugs used in malaria chemoprevention. The main goal of the technical consultation was to agree on the most important PPCs for drugs t
...
Although Shiga toxin-producing Escherichia coli (STEC) have been isolated from a variety of food production animals, they are most commonly associated with ruminants from which we derive meat and milk. Because of the widespread and diverse nature of ruminant-derived food production, coupled with the
...
Trypanosoma cruzi is the etiological agent of Chagas disease (CD), considered one of the most important parasitic infections in Latin America. Between 25 and 90 million humans are at infection risk via at least one of multiple infection mechanisms. Under natural conditions, the principal transmissio
...
This document compiles the recommendations made by the World Health Organization (WHO) and the Pan American Health Organization (PAHO) to help professionals in charge of vector control programs in Latin America and the Caribbean at the national, subnational, and local level update their knowledge in
...
El presente documento reúne un conjunto de recomendaciones formuladas por la Organización Mundial de la Salud (OMS) y la Organización Panamericana de la Salud (OPS) para ayudar, a los profesionales encargados de los programas de control de vectores de Latinoamérica y el Caribe a nivel nacional,
...