Filter
1098
Featured
167
353
Language
Document type
658
221
96
51
34
15
10
9
2
1
1
Countries
94
41
39
38
38
35
28
26
25
23
23
23
19
18
18
15
14
13
13
13
12
11
10
9
9
7
7
6
6
6
5
5
5
5
5
4
4
4
4
4
4
4
4
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
Publication Years
Category
499
134
56
43
18
15
12
Toolboxes
128
124
104
82
57
57
36
16
15
15
13
11
10
8
7
4
4
4
3
3
1
PQDx 0159-055-00
WHO PQ Public Report
February/2017, version 5.0
The following protocol has been designed to investigate the First Few X cases (FFX) and their close contacts. It is envisioned that the FFX 2019-nCoV investigation will be conducted across several countries or sites with geographical and demographical diversity. Using a standardized protocol such a
...
It is intended for clinicians who are working in intensive care units (ICUs) in low and middle-income countries and managing adult and pediatric patients with severe forms of acute respiratory infection (SARI), including severe pneumonia, acute respiratory distress syndrome (ARDS), sepsis and septic
...
COVID-19: Guidelines for case-finding, diagnosis, management and public health response in South Africa
recommended
The information contained in this document, be it guidelines, recommendations, diagnostic algorithms or treatment regimens, are offered in this document in the public interest. To the best of the knowledge of the guideline writing team, the information contained in these guidelines is correct. Imple
...
Methicillin-resistant Staphylococcus aureus(MRSA) strainsor multidrug-resistant S.aureus, initially described in 1960s,emerged in the last decade as a cause of nosocomial infections responsible for rapidly progressive, potential fatal diseases including life-threatening pneumonia, necrotizing fascii
...
Primary Care Guide for the Adult· 2020
Western Cape Edition
20 March 2020
The following protocol has been designed to investigate the extent of infection, as determined by seropositivity in the general population, in any country in which COVID-19 virus infection has been reported. Each country may need to tailor some aspects of this protocol to align with public health, l
...
First published in 2020, this toolkit is intended for clinicians working in acute care, managing adult and paediatric patients with acute respiratory infection, including severe pneumonia, acute respiratory distress syndrome, sepsis and septic shock. The main objective is to provide key tools for us
...
The following is a breakdown of key considerations to guide planning and resource allocation for COVID-19 preparedness and response to support UNHCR regional and country operations in Public Health, WASH, Shelter and Settlements programmes. While some of the activities are more relevant in camps or
...
The number of confirmed COVID-19 cases detected and reported in each country is influenced by
many factors including limited access and/or utilization of healthcare and COVID-19 testing, limited
surveillance, lack of knowledge amongst the population about when to seek testing, an asymptomatic pres
...
Guidance on pooled testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
recommended
The purpose of this document is to provide guidance on the use of pooled sample testing strategy in coronavirus disease (COVID-19)
testing laboratories of the African Union Member States for scaling up SARS-CoV-2 nucleic acid testing capacity with the available resources. The current document descr
...
Available in English, French, Spanish, Chinese, Russian and Arabic
https://apps.who.int/iris/handle/10665/337832
These guidelines provide new and updated recommendations on the use of point-of-care testing in children under 18 months of age and point-of-care tests to monitor treatment in people living with HIV; the treatment monitoring algorithm; and timing of antiretroviral therapy (ART) among people living w
...
Interim Guidance October 2022. This addendum addresses some of the methodological aspects of VE evaluations that have been learned during the past year, as well as those that have become relevant in the current epidemiological setting of the COVID-19 pandemic. For some of the COVID-19 vaccine method
...
Epidemic meningitis is a major public health challenge in the African 'meningitis belt', an area that extends from Senegal to Ethiopia with an estimated total population of 500 million. Since 2002, the World Health Organization (WHO), in collaboration with its collaborating centres for meningitis, h
...
3rd edition. In 2001, Uganda adapted the Integrated Disease Surveillance and Response (IDSR) developed by World Health Organization (WHO) for member states in African region. The Ministry of Health has been implementing the IDSR strategy since then with success across the country. This strategy prov
...
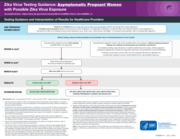
Testing Guidance and Interpretation of Results for Healthcare Providers
Zika Virus Testing Guidance: Asymptomatic Pregnant Women
with Possible Zika Virus Exposure