Filter
182
Featured
44
41
Language
Document type
63
56
25
18
9
5
2
2
1
1
Countries
10
4
3
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
Publication Years
Toolboxes
40
9
8
7
4
4
2
2
2
1
1
Updated recommendations on treatment of adolescents and children with chronic HCV infection
recommended
The new treatment recommendations that extend the 2018 treat all recommendation for adults with chronic HCV infection to include adolescents and children down to 3 years, and to align the existing recommended pangenotypic direct-acting antiviral (DAA) regimens (SOF/DCV, SOF/VEL and G/P) for adults,
...
WHO’s Dr Philippa Easterbrook gives a situation update on the recent hepatitis outbreak affecting children including possible causes and steps parents, caregivers and countries should take.
manual de referencia rápida de anomalías congénitas e infecciones seleccionadas
These guidelines provide updated evidence-based recommendations on the priority HCV-related topics from the 2018 WHO Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C infection and the 2017 WHO Guidelines on hepatitis B and C testing. These priority areas are:
...
WHO’s Essential Medicines List and List of Essential Diagnostics are core guidance documents that help countries prioritize critical health products that should be widely available and affordable throughout health systems. The updated Essential Medicines List adds 23 medicines for children.
Series Quality of Life for Children with Cancer: Modules on Paediatric Palliative Care. Module 5: Caring for the Caregiver
Abridged version. In this abridged version of the Evidence-based Clinical Practice Guidelines for the Follow-Up of at-risk neonates, we provide recommendations for the care of newborns up to 2 years of age, corresponding to the first phase of their follow-up. The recommendations are intended for all
...
Handbook of Hospital Care for Newborn Infants
recommended
Updated June 2021
The paper provides the rationale for these recommendations, which are based on analyses of data from the TRACT trial.
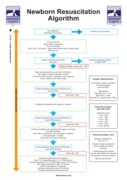
Newborn Resuscitation Algorithm
Quality of Life for Children with Cancer Series: Modules on Paediatric Palliative Care. Module 8: End-of-Life Care
Quality of Life for Children with Cancer Series: Modules on Paediatric Palliative Care. Module 7: Spirituality
Quality of Life for Children with Cancer Series: Modules on Paediatric Palliative Care. Module 1: Overview of paediatric palliative care
Quality of Life for Children with Cancer Series: Modules on Paediatric Palliative Care. Module 4: Symptom Management
Quality of Life for Children with Cancer Series: Modules on Paediatric Palliative Care. Module 2: Care at Home