Filter
550
Featured
Recommendations
140
New Publications
3
Language
Document type
Guidelines
283
Fact sheets
90
Studies & Reports
55
Manuals
41
Infographics
22
Videos
14
Resource Platforms
13
Online Courses
13
Training Material
10
Situation Updates
5
Brochures
2
No document type
2
Countries / Regions
Russia
50
Brazil
44
Germany
29
Latin America and the Carribbean
27
Middle East and North Africa
22
Mozambique
21
Angola
21
West and Central Africa
21
Guinea-Bissau
16
East and Southern Africa
16
Africa
13
Global
11
Syria
10
Yemen
10
Eastern Europe and Central Asia
10
Spain
9
South Africa
7
Ecuador
5
Western and Central Europe
5
Eastern Europe
5
India
4
South Sudan
3
Argentina
3
Western Pacific Region
3
Vietnam
3
France
3
Nigeria
2
Haiti
2
Philippines
2
Sudan
2
Ukraine
2
South–East Asia Region
2
Kazakhstan
2
Portugal
2
Burkina Faso
1
Senegal
1
Mali
1
Côte d’Ivoire / Ivory Coast
1
Saudi Arabia
1
Ethiopia
1
Cameroon
1
Niger
1
Togo
1
Somalia
1
Nepal
1
Turkey
1
Chad
1
Cambodia
1
China
1
Bangladesh
1
Benin
1
Central African Republic
1
Austria
1
Switzerland
1
Burundi
1
El Salvador
1
Bolivia
1
Madagascar
1
Luxembourg
1
Venezuela
1
Italy
1
Djibouti
1
Laos
1
Congo-Brazzaville
1
Canada
1
Belgium
1
United Kingdom
1
Authors & Publishers
Publication Years
Category
Countries
224
Clinical Guidelines
31
Pharmacy & Technologies
21
Women & Child Health
16
Capacity Building
9
Key Resources
4
Public Health
2
4 march 2022
What have we learned about the symptoms of Long COVID or Post COVID-19 condition so far? How long does it last, when should you worry, and what treatments are recommended? WHO’s Dr Janet Diaz explains in Science in 5.
May 9, 2022.Since the onset of the COVID-19 pandemic, a large number of clinical trials have been planned and developed to assess the effectiveness and safety of various interventions that could prevent hospitalizations and progression to severe disease in people infected with SARS-CoV-2. Currently,
...
11 May 2022
What are WHO's recommendations for COVID-19 vaccines and children? And what does the evidence say so far about the safety of these vaccines in children? And if you live in a country where this vaccination is not available for your kids, how can you keep them safe? WHO’s Dr Soumya Swa
...
Curr HIV/AIDS Rep. 2022; 19(1): 37–45.
Published online 2022 Jan 29. doi: 10.1007/s11904-021-00589-4.
COVID-19 has had an unprecedented impact on the cascade-of-care among PLWH in LAC. There is a need for
longitudinal studies that assess clinic implication of these pandemic interactions in LAC.
...
Multiple countries are reporting severe acute cases of hepatitis of unknown aetiology in children, in several
regions of the world. WHO has developed this clinical case report form (CRF) to support and facilitate reporting
of anonymized, patient-level data of acute hepatitis of unknown aetiology.
This document provides interim guidance to countries on testing considerations and strategies for suspect cases of severe acute hepatitis of unknown aetiology in children. It is primarily intended for clinical, programmatic, laboratory and diagnostic stakeholders across Member States and national pu
...
Hepatitis aguda de etiología desconocida en niños: Orientaciones provisionales para los laboratorios
Este documento ofrece una orientación provisional a los países sobre las consideraciones y estrategias de análisis para los casos sospechosos de hepatitis aguda grave de etiología desconocida en niños. Está dirigido principalmente a las partes interesadas en el ámbito clínico, programático,
...
This document aims to provide interim guidance for microbiology and virology experts, other laboratory professionals, laboratory managers, infectious disease programme managers, public health professionals and other stakeholders that provide primary, confirmatory or advanced testing for SARS-CoV-2,
...
Revised COVID-19 Testing Strategy
recommended
Second edition. June 2022. This revised guidance recommends that access to COVID-19 testing is decentralized as far as possible and made available at health facilities, and through the use of self tests to enable access to care and the mitigation of transmission. Testing should be prioritized for hi
...
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
Nirmatrelvir-ritonavir for COVID-19
recommended
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
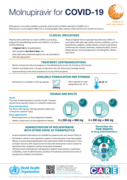
Molnupiravir for COVID-19
recommended
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
Administration of Molnupiravir for COVID-19
recommended
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
Remdesivir for COVID-19
recommended
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.